Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Okubo, Takahiro*; Ibaraki, Moe*; Tachi, Yukio; Iwadate, Yasuhiko*
Applied Clay Science, 123, p.148 - 155, 2016/04
Times Cited Count:29 Percentile:74.44(Chemistry, Physical)The pore distribution of water-saturated compacted clay (Na-montmorillonite at 0.8 and 1.4 g/cm saturated by three salt concentrations) was evaluated using
saturated by three salt concentrations) was evaluated using  H NMR relaxometry and freezing point depression. The populations of interlayer water with four hydrated state and non-interlayer water were calculated from the assumed thresholds. The sample with lower density exhibits higher population of non-interlayer water up to 55%. Low-temperature
H NMR relaxometry and freezing point depression. The populations of interlayer water with four hydrated state and non-interlayer water were calculated from the assumed thresholds. The sample with lower density exhibits higher population of non-interlayer water up to 55%. Low-temperature  H NMR experiments in view of freezing point depression indicated that mesopore water in approximately 4 nm space observed in the calorimetric study was considered as non-interlayer water and the threshold temperature. The result showed that population of non-interlayer water by expected from freezing point depression agreed with
H NMR experiments in view of freezing point depression indicated that mesopore water in approximately 4 nm space observed in the calorimetric study was considered as non-interlayer water and the threshold temperature. The result showed that population of non-interlayer water by expected from freezing point depression agreed with  H NMR relaxometry within 10%. Correlation experiments between longitudinal (
H NMR relaxometry within 10%. Correlation experiments between longitudinal (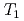 ) and transverse relation times (
) and transverse relation times (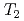 ) at -10
) at -10 C suggested that high-mobility bulk-like water molecules existed at a clay density of 1.4 g/cm
C suggested that high-mobility bulk-like water molecules existed at a clay density of 1.4 g/cm .
.
Nagao, Seiya; Yanase, Nobuyuki; Yamamoto, Masayoshi*; Kofuji, H.*; Sorin, Yoshiki*; Amano, Hikaru
Journal of Radioanalytical and Nuclear Chemistry, 252(2), p.225 - 232, 2002/05
Times Cited Count:9 Percentile:51.46(Chemistry, Analytical)no abstracts in English
Nagao, Seiya*;
Science of the Total Environment, 117-118, p.439 - 447, 1992/00
no abstracts in English
*; ; *; *
Kozan Chishitsu, 40(5), p.323 - 336, 1990/00
no abstracts in English
 Pellet
PelletJAERI-M 7158, 23 Pages, 1977/07
no abstracts in English
Tachi, Yukio; Yotsuji, Kenji; Ito, Tsuyoshi; Suyama, Tadahiro
no journal, ,
The integrated sorption and diffusion (ISD) model was applied for systems coexisting multispecies Sr (divalent cation Sr and neutral SrSO
and neutral SrSO (aq)) in compacted montmorillonite. Effective diffusion coefficients (De) and distribution coefficients (Kd) of Sr in compacted Na-montmorillonite (dry density of 800 kg/m
(aq)) in compacted montmorillonite. Effective diffusion coefficients (De) and distribution coefficients (Kd) of Sr in compacted Na-montmorillonite (dry density of 800 kg/m ) saturated with three types of Na
) saturated with three types of Na SO
SO solutions (0.05, 0.1, 0.5 M) were measured by the trough-diffusion method. The De and Kd values decreased drastically with increasing porewater salinity. The De for multispecies Sr was determined as the harmonic weight-average considering the two species distribution and their log De values, based on comparison with reactive-transport calculations using the PHREEQC. As a result, the De trend could be quantitatively express by the ISD model considering multispecies contributions. The thermodynamic sorption model considering ion exchange reactions could provide reasonable account of Kd trend as functions of salinity.
solutions (0.05, 0.1, 0.5 M) were measured by the trough-diffusion method. The De and Kd values decreased drastically with increasing porewater salinity. The De for multispecies Sr was determined as the harmonic weight-average considering the two species distribution and their log De values, based on comparison with reactive-transport calculations using the PHREEQC. As a result, the De trend could be quantitatively express by the ISD model considering multispecies contributions. The thermodynamic sorption model considering ion exchange reactions could provide reasonable account of Kd trend as functions of salinity.